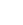Molecular Structure and Synthesis of 2,3-Dimethyl-2,3-Diphenylbutane
Introduction:
2,3-Dimethyl-2,3-diphenylbutane is a compound with an intriguing molecular structure that has garnered significant attention in organic chemistry. This article aims to explore its molecular structure, synthesis, and potential applications.
Molecular Structure:
2,3-Dimethyl-2,3-diphenylbutane is a symmetrical hydrocarbon with the molecular formula C20H22. It consists of a butane backbone with two phenyl groups attached to the 2 and 3 carbon positions, and two methyl groups attached to the same carbons. This arrangement gives rise to its unique three-dimensional shape, contributing to its chemical properties and reactivity.
Synthesis:
The synthesis of 2,3-dimethyl-2,3-diphenylbutane involves several steps, starting from readily available starting materials. One common synthetic route begins with the alkylation of phenylacetonitrile with bromopropane, followed by reduction of the resulting nitrile to obtain 2,3-dimethyl-2,3-diphenylbutanenitrile. The final step involves the hydrolysis of the nitrile group to yield the desired product.
Alternatively, the compound can be synthesized via a Friedel-Crafts alkylation reaction. This involves the reaction of benzene with 3-methyl-2-bromopropene using a Lewis acid catalyst. The resulting intermediate can then be converted into 2,3-dimethyl-2,3-diphenylbutane through further manipulations.
Applications:
2,3-Dimethyl-2,3-diphenylbutane possesses interesting properties that make it valuable in various applications. Its bulky structure and steric hindrance can influence its reactivity and selectivity in chemical reactions. Additionally, the presence of aromatic rings can impart unique properties, such as increased rigidity and electron delocalization, which can be exploited in different fields.
One potential application lies in the field of organic synthesis, where this compound can serve as a building block for the construction of more complex molecules. Its unique structure can introduce new functionalities and spatial arrangements, enabling the creation of diverse chemical architectures.
Furthermore, 2,3-dimethyl-2,3-diphenylbutane may find utility in the development of novel materials. Its structural features can contribute to enhanced mechanical properties, such as increased strength or rigidity, making it a potential candidate for use in polymers or composite materials.
Conclusion:
In conclusion, 2,3-Dimethyl-2,3-diphenylbutane exhibits a fascinating molecular structure that can be synthesized through various routes. Its unique properties and reactivity make it an intriguing compound with potential applications in organic synthesis and material science. Further research and exploration of this compound could uncover additional uses and unlock its full potential in various fields of chemistry and beyond.